
TCS Bioscience는 생명 과학 및 진단 산업을 위한 고품질 혈청, 항원, 항체 및 기타 생물학적 제품을 제공하는 글로벌 바이오테크 기업입니다.
제품 설명
PliNOSa® Test Plasma iNOS EIA
제품 번호
Z5-K-1600
제품 특징
Product Code: Z5-K-1600
CI ID: 5612
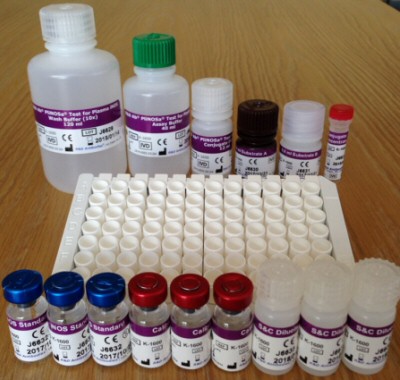
Pack Size: Kit
Typical Lead Time: Approximately 10 working days from receipt of order. Please contact our Customer Services Team if you have any queries.
TCS Biosciences Ltd is pleased to announce our partnership with R&D Antibodies. R&D Antibodies has delivered high quality research antibodies and immunoassays to the life sciences and research markets since 1984.
The PliNOSa® K-1600 Assay Kit is a novel in vitro diagnostic immunoassay for the early identification of sepsis.
The PliNOSa® test detects inducible Nitric Oxide Synthase (iNOS) on microvesicles in plasma, an innovation in sepsis disease pathology.
The PliNOSa® test can help physicians make better and more informed choices for improved patient management.
iNOS has specifically been implicated in sepsis, severe sepsis and septic shock. The PliNOSa® K-1600 assay kit provides accurate measurement of circulating iNOS concentrations in plasma samples via a chemiluminescent sandwich EIA. Each kit is for 96 tests.
PliNOSa® features
Test benefits
The kit includes:
| Table 1 - Organ Dysfunction Associated with Sepsis in Severely Injured Trauma Patients | |||
| N = 187 | Heart, Lung or Kidney Dysfunction | ||
| Present | Absent | ||
| iNOS | Positive | 107 | 5 |
| Negative | 15 | 60 | |
| Sensitivity = 88% | PPV = 96% | ||
| Specificity = 92% | NPV = 80% | ||
Until now, sepsis has been an intractable disease that has baffled medical researchers for centuries.
Biochemical markers such as procalcitonin (PCT), IL-6, lactate and CRP have lacked the necessary specificity and sensitivity. These assays often fail to help physicians and clinicians to identify at-risk patients who are progressing down the sepsis pathway.
The PliNOSa® test helps physicians assess a patient's risk of developing sepsis as much as 48 hours before recognisable symptoms appear.
TCS Biosciences의 모든 제품을 만나 보세요!
Accu-Bac Quantitative Micro-organisms
Contract In Vitro Diagnostics Manufacturing
Environmental Monitoring and Sample Collection
Bacteria and Biofilm Detection Systems
Proteins (Caf1-Fibronectin) and (Caf1-Vitronectin)
Microbiological Quality Control Organisms
PliNOSa® K-1600 Assay Kit - An Innovation for Sepsis Screening
TCS Biosciences - Official Distributor in South Korea "Morebio" 한국 공식 대리점 "모아바이오"