
YEASEN은 생명과학 연구 및 진단을 위한 고품질의 연구용 시약과 기기를 제공하는 글로벌 바이오 기업입니다.
제품 설명
LZCap AG (3'Acm) GMP-Grade, High Affinity (100mM)
제품 번호
10684ES
제품 설명
Cap AG (3'Acm) is a cap analog with the structure of m7(3'AcmG)(5')ppp(5')(2'0MeA)pG, the molecular formula of it is C35H48N16O24P4 and the molecular weight is 1200.75. The product is used for transcription with the initial sequence of 5'AG3', and the natural cap1 structure is produced by Cap1 transcription capping. Compared with Cap0 produced by traditional capping method, the cap1 structure produced by CapAG (3'Acm) enables the mRNA to have higher activity and translation efficiency in vivo.
Feature
Cat No. | Name | Size |
10684 | LZCap AG (3'Acm) (100mM) | 100 μL |
1 mL |
Molecular Formula | C35H48N16O24P4 (Free acid) |
Molecular weight | 1200.75(Free acid) |
Concentration | 100± 3 mM |
Purity | HPLC ≥95% |

Figure 1. LZCap analog showed stronger affinity to translation initiation factors eIF4E, allowing more efficient initiation of mRNA translation. The lower the Kd value, the stronger the affinity of the cap analog to the cap-binding protein eIF4E.

Figure 2. In vivo expression of LZCap AG capped mRNA is much higher than that of 3’OMe cap analog.
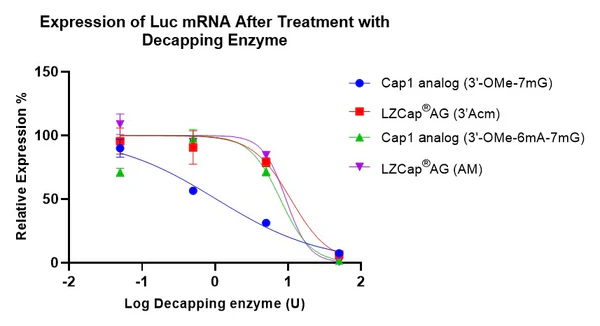
Figure 3. LZCap capped mRNAs are more resistant to the decapping enzyme. The higher the IC50 value, the stronger the ability of the cap analog to resist cleavage by the decapping enzyme
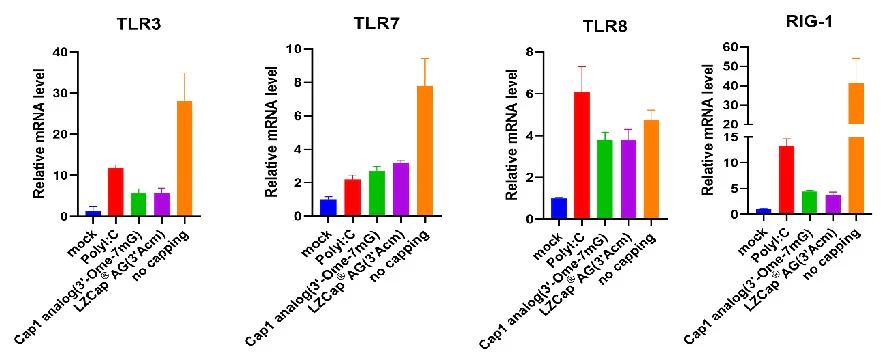
Figure 4. LZCap capped mRNA reduces single RNA-stimulated immune factor transcription level in THP-1 cell.
Study | Result and Conclusion |
Cytotoxicity Test | No cytotoxicity was observed with the modified nucleoside in multiple cell lines |
Polymerase Inhibition Study | The modified nucleoside is neither an inhibitor nor a substrate of human RNA and DNA polymerases and is therefore not integrated into the genome |
Ames Test* | No genotoxicity was observed in the Ames test |
This product could be stored at -25~-15 ℃ for two years.
1. How do you design the LZCap?
Enzymes have a relatively "specific" recognition of substrates. Therefore, when designing a new cap structure, on one hand, we need novel structural modifications for patent purposes, but on the other hand, we strive to maintain similarity with natural/known structures as much as possible. The natural structure has a ribose 3' OH, which can be modified (e.g., methylation). Based on this consideration, we chose to add a carbon at the 3' position for patent novelty, followed by an NH to mimic the hydrogen bonding of OH, and then an acetyl group to reduce the basicity of NH and enhance its hydrogen bonding capability. The activity of LzCap is better than methylated natural cap, possibly due to increased hydrogen bonding. Compared to the methyl and methoxy groups, the acetyl amino group may also increase van der Waals interactions between the substrate (cap) and the initiating factor (enzyme).
2. Is the acetyl amino group stable?
The acetyl amino group is already sufficiently stable. It is much more stable than the 7-methylated position and the phosphodiester bond, which are the least stable parts of the cap.
YEASEN의 모든 제품을 만나 보세요!
YEASEN - Official Distributor in South Korea "Morebio" 한국 공식 대리점 "모아바이오"