Proteogenix는 Primary Antibody Biosimilar - Research Grade
제품을 전문적으로 생산하는 20년의 경험이 있는 High Quality 제조사입니다.
제품 설명
제품 번호
PX-COV-P061
제품 특징
| Product name | SARS-CoV-2 RBD of Spike protein, L452R, T478K – lineage B.1.617.2 – Indian Delta Variant |
|---|---|
| Origin species | SARS-COV2 |
| Expression system | Eukaryotic expression |
| Sequence | YP_009724390.1 |
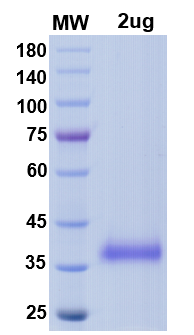
| Molecular weight | 35kDa |
| Buffer | PBS, pH7.5 |
| Form | Liquid |
| Delivery condition | Dry Ice |
| Storage condition | 4°C for short term; -20°c or -80°C for long term |
| Brand | ProteoGenix |
| Host species | Mammalian cells |
| Applications | ELISA,WB |
| Fragment Type | Spike protein fragment |
| Aliases /Synonyms | B.1.617.2, VOC21APR-02, G/452R.V3, Indian variant, Delta variant |
| Reference | PX-COV-P061 |
| Note | For research use only. Not suitable for human use. |
There are currently three circulating clades of lineage B.1.617 – B.1.617.1, B.1.617.2, and B.1.617.3, shown in chronological order of detection. Although these clades display different mutation profiles, all were initially detected in India and have since spread to other continents. Of the three clades, lineage B.1.617.2 carrying receptor-binding domain mutations L452R and T478K is the one causing more concern among the scientific community and governments.
Public Health England (PHE) has recently escalated lineage B.1.617.2 from variant under investigation (VUI) to variant of concern – VOC21APR-02 – due to the steep increase of cases in the UK. In early May 2021, the number of cases of this variant with a travel history in India already represented a minority of the total amount of B.1617.2 cases detected in the country. Data suggests a sustained spread of the new variant and hints to its higher infectivity in comparison to other circulating variants of SARS-CoV-2. Although the absolute number of cases remains low, the spread rate of this variant is very high. These findings suggest variant B.1.617.2 may possess transmissibility similar to that of B.1.1.7, one of the most abundant variants of the virus circulating in the UK since September 2020. But it is still unclear if this variant will outcompete the UK strain.
Mutation L452R has been previously detected in two other high abundance variants circulating in the US (lineages B.1.427 and B.1.429). This mutation has been associated with weaker neutralization of the virus by convalescent plasma. However, the available data is still insufficient to understand how this amino acid change might affect vaccine efficiency. Less data is available on the effects of mutation T478K. This amino acid change occurs in the region of interaction between the virus and the human ACE2 receptor. Several lineages of SARS-CoV-2 have been shown to carry this mutation, namely lineage B.1.1.519 (US/Mexico lineage). Moreover, several reports indicate that its abundance among circulating variants has shown a marked increase since January 2021. To date, there are no reports regarding the effects of this change on the transmissibility and immune evasion ability of SARS-CoV-2. For this reason, more functional studies are necessary to determine the potential effect of these combined RBD mutations on COVID-19 vaccine and drug efficiency.
QC & Validation Data
Publications
- Richel E, Wagner JT, Klessing S, Di Vincenzo R, Temchura V and Überla K (2023) Antigen-dependent modulation of immune responses to antigen-Fc fusion proteins by Fc-effector functions. Front. Immunol. 14:1275193. doi: 10.3389/fimmu.2023.1275193
- Hubert Bernauer, Anja Schlör, Josef Maier, Norbert Bannert, Katja Hanack, Daniel Ivanusic, tANCHOR fast and cost-effective cell-based immunization approach with focus on the receptor-binding domain of SARS-CoV-2, Biology Methods and Protocols, Volume 8, Issue 1, 2023, bpad030, https://doi.org/10.1093/biomethods/bpad030
- Winklmeier S, Rübsamen H, Özdemir C, Wratil PR, Lupoli G, Stern M, Schneider C, Eisenhut K, Ho S, Wong HK, Taskin D, Petry M, Weigand M, Eichhorn P, Foesel BU, Mader S, Keppler OT, Kümpfel T and Meinl E (2024) Intramuscular vaccination against SARS-CoV-2 transiently induces neutralizing IgG rather than IgA in the saliva. Front. Immunol. 15:1330864. doi: 10.3389/fimmu.2024.1330864
ProteoGenix의 모든 제품들을 만나보세요!
Proteogenix - Exclusive Distributor in South Korea "Morebio" 한국 독점 대리점 "모아바이오"