
YEASEN은 생명과학 연구 및 진단을 위한 고품질의 연구용 시약과 기기를 제공하는 글로벌 바이오 기업입니다.
제품 설명
MycAway™ Mycoplasma qPCR Detection Kit (2G)
제품 번호
40619ES
제품 설명
dsDNA BR Assay Kit is a simple, sensitive, accurate and wide range fluorescence quantification kit for double-stranded DNA (dsDNA BR) with good linearity in the range of 2~1000 ng. The kit contains fluorescence detection reagent, buffer and related dsDNA BR standards. Before use, the fluorescence detection reagent is diluted into working solution with buffer, then the dsDNA sample to be measured is added, a standard curve is made, and readings are performed using a fluorescent enzyme marker or Qubit™ fluorometer. The product has good tolerance to conventional contaminants such as proteins and salts.
Fast & Convenient: Sample prep and testing completed in under 3 hours, versus 28 days for culture(Alternative to the standard 28-day culture test).
High Sensitivity: Detects as low as 10 CFU/mL, making it a reliable alternative to culture methods.
High Specificity: 16S rRNA-based primers and probes avoid cross-reactivity with related species such as Clostridium and Sesamum.
Safe to Use: Non-infectious positive control eliminates contamination risk.
Strong Anti-Interference: Internal control (IC) detects sample inhibition or reaction errors, reducing false negatives.
Regulatory Compliance: Validated according to the requirements of EP2.6.7, JP G3 and USP 63 pharmacopoeia, in line with the standards of international authorities.
Sample Type | media, cells, raw materials; Biopharmaceutical Purification Sample, Bulk Drug Substance Sample, Cell Cultures. |
Detect method | qPCR method(Taqman fluorescent) |
Detect Time | <4 hours |
Fluorescent probe | FAM(Target channel);VIC(Internal channel) |
Pretreatment kit | Compatible with magnetic bead-based sample pretreatment kits(Cat#18461) |
Applicable Models | Thermo: ABI7500, ABI QuantStudio™ 5; Roche: LC 480; Bio-Rad:CFX96 Optic Module |
Detection Limit(LOD) | 10 CFU/mL |
Covered mycoplasma | 183 mycoplasma species |
Validation | Validated according to EP <2.6.7> |
Components No. | Name | 40619ES25 | 40619ES60 |
40619-A | 2×MyqPCR Reaction Buffer | 375 μL | 1.5 mL |
40619-B | MyPrimer & Probe Mix | 100 μL | 400 μL |
40619-C* | Internal Control (IC) | 25 μL | 100 μL |
40619-D** | Positive Control (PCS) | 250 μL | 1 mL |
40619-E*** | DNA Dilution buffer | 500 μL | 2×1 mL |
40619-F**** | Ultrapure water | 500 μL | 2×1 mL |
[Note]: *IC: Internal control.
**PCS: Positive control solution,the concentration is 1,000 copies/µL.
***DNA Dilution buffer: used for IC dilution and the template of NTC and NCS.
****Ultrapure water: used for the preparation of qPCR Mix.
This product should be stored at -25~-15℃ for 2 years.
*Upon receipt of the kit, please check whether all components are complete and immediately store them in -25~-15℃ condition if not perform the assay immediately. Please note 40619-B should be stored away from light.
Mycoplasma Detection; Mycoplasma Detection for BioProduction process media, cells, raw materials.
1. Workflow
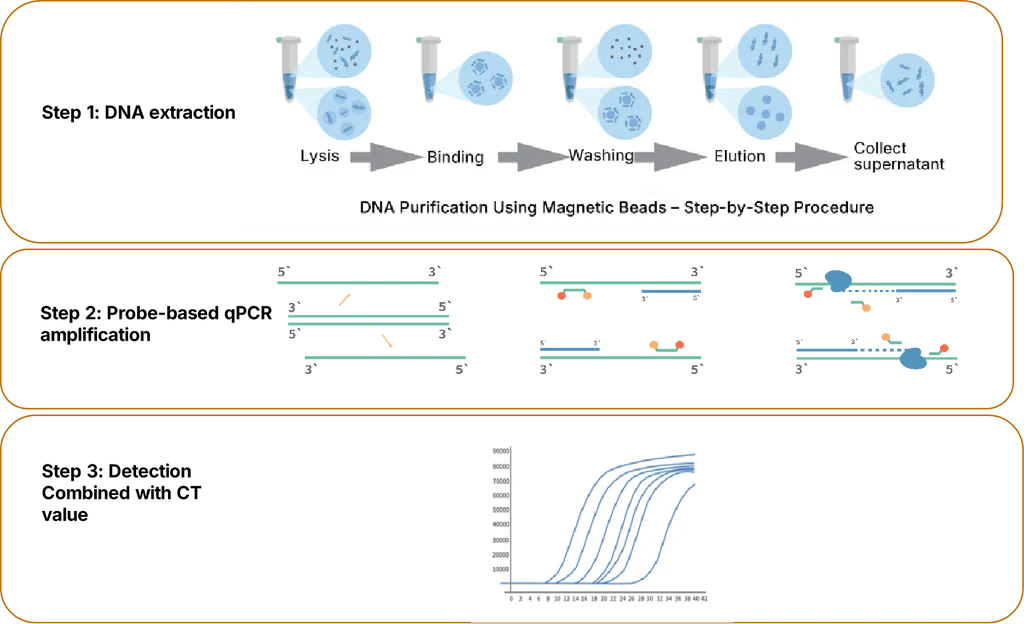
Figure 1. Workflow of mycoplasma detection using the Mycoplasma Real-time Quantitative PCR Detection Kit
2. Specificity
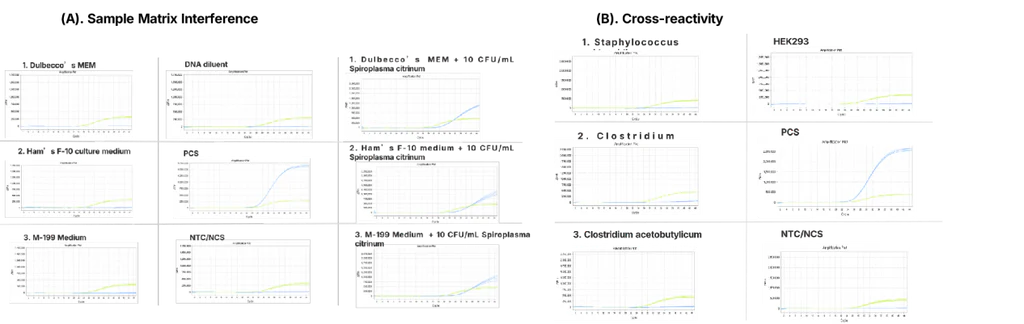
Figure 2. Specificity validation.
(A). Nine common sample matrices and the kit’s DNA dilution buffer showed no mycoplasma detection in the target channel (FAM, blue). Normal FAM signal was observed only when Mycoplasma fermentans was added to a sample matrix.
(B). DNA from 14 non-Mycoplasmatales strains and 6 commonly used biopharmaceutical cell lines showed no target channel amplification. In all cases, the internal control channel (Cy5, green) amplified normally.
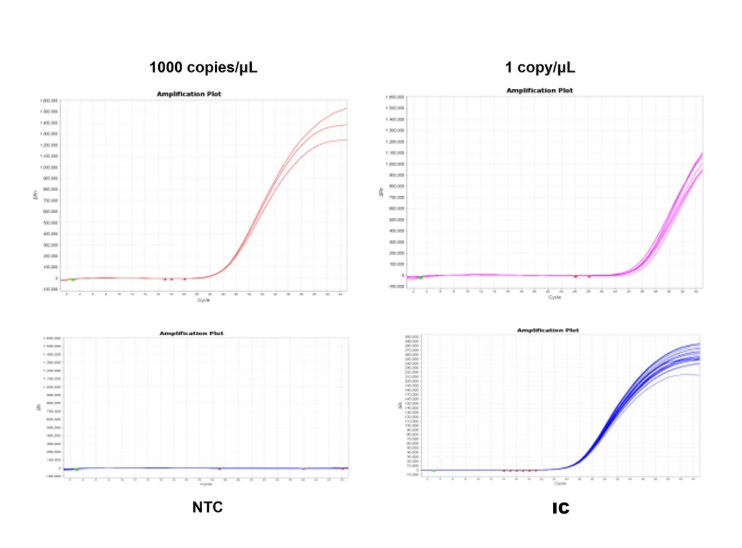
3. Limit of Detection (LOD): >95% detection rate.
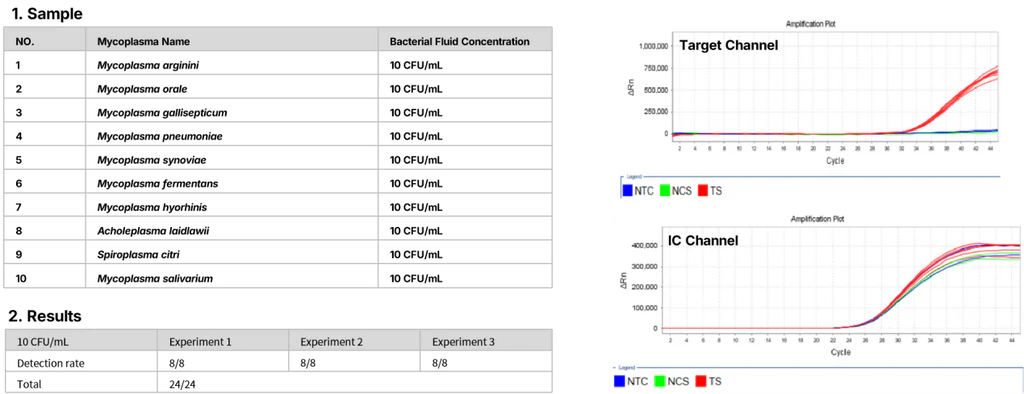
Figure 3. Limit of detection (LOD) test.
Ten mycoplasma standard strains (10 CFU/mL), sourced from German MB, were tested following kit instructions for nucleic acid extraction(Cat#18461) and detection kit(Cat#40619). Mycoplasma samples were tested three times with 8 replicates each. Each run included NCS (negative control solution) and NTC (no template control).
When controls passed, at least 23 out of 24 replicates tested positive for each strain.
YEASEN의 모든 제품을 만나 보세요!
YEASEN - Official Distributor in South Korea "Morebio" 한국 공식 대리점 "모아바이오"