Biotechrabbit은 바이오의약품 및 생명과학 연구를 위한 고급 실험 재료와 서비스를 제공하는 회사입니다.
제품 설명
Tris-NTA Amine
제품 번호
BR1001101 - 100µg
BR1001102 - 1mg
제품 특징
Description
His-tags are one of the most commonly used tags for protein expression analysis. Conventional metal ion chelators, such as nitrilotriacetic acid (NTA) and iminodiacetic acid (IDA), bind His-tags with low affinities in the range of 10 µM. The biotechrabbit Tris-NTA complexes three NTA groups that together bind a 6×His-tag with an affinity that is four orders of magnitude higher (1 nM) than is possible with conventional chelators (10 µM). The binding of His-tags is stoichiometric and single-molecule detection is possible. Binding is reversible: bound His-tags can be released with imidazole or ethylenediaminetetraacetic acid (EDTA).
biotechrabbit™ Tris-NTA is available with a free amine group or conjugated to biotin. It can be used in a large range of applications, including protein detection and labeling, coupling proteins, lipids or cells to surfaces, protein purification and reversible protein modification.
NOTICE TO PURCHASER: LIMITED LICENSE
The use of this product for research purposes is covered by a license from QIAGEN Group. No rights to use this product to perform or offer diagnostic, companion diagnostic, commercial testing or other commercial services for money or money’s worth are granted by the supply of this product expressly, by implication or by estoppel.
Should you wish to use this product for any other purpose not covered by this license, please contact QIAGEN Corporate Business Development at bd@qiagen.com.
Other Information
Quality ControlIdentityIdentity of the substance was determined by MS analysis. The identity was consistent with the reference substance. PurityThe purity of Tris-NTA Amine was determined by HPLC. Purity was >95%. |
Protocols
Please, choose the metal ion (i.e. Nickel or Cobalt) and loading method that fit best to your application.
As an example, please, refer to Figure 5 in "Reichel et al., Anal Chem., 2007, 79, 8590–600":
Ni2+ loading by injection of 10 mM NiCl2 when using Tris-NTA with a biosensor surface.
High-Affinity Adaptors for Switchable Recognition of Histidine-Tagged Proteins.
Lata et al., J. Am. Chem. Soc., 2005, 127, 10205–10215
Specific and Stable Fluorescence Labeling of Histidine-Tagged Proteins for Dissecting Multi-Protein Complex Formation.
Lata et. al., J. Am. Chem. Soc., 2006, 128, 2365–2372
Noncovalent, Site-Specific Biotinylation ofHistidine-Tagged Proteins.
Reichel et al., Anal Chem., 2007, 79, 8590–600
Identifying Modulators of Protein-Protein Interactions Using Photonic Crystal Biosensors.
Heeres et al., J Am Chem Soc. 2009, 131: 18202–18203
Tris-Nitrilotriacetic Acids of Sub-nanomolar Affinity Toward Hexahistidine Tagged Molecules.
Huang et al., Bioconjug Chem., 2009, 20: 1667–1672
Four-color single-molecule fluorescence with noncovalent dye labeling to monitor dynamic multimolecular complexes.
DeRocco et al., BioTechniques 2010, 49: 807-816
Co- and distinct existence of Tris-NTA and biotin functionalities on individual and adjacent micropatterned surfaces generated by photo-destruction.
Biswas et al., Soft Matter, 2014, 10, 2341–2345
High-affinity gold nanoparticle pin to label and localize histidine tagged protein in macromolecular assemblies.
Anthony et al., Structure, 2014, 22: 628–635
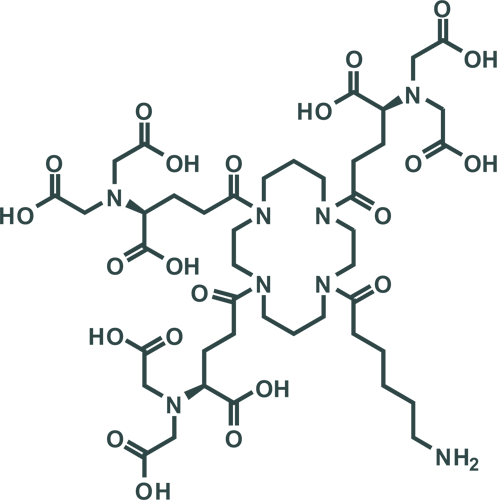 |
Biotechrabbit에서 다양한 제품을 찾아보세요!
Biotechrabbit - Official Distributor in South Korea "Morebio" 한국 공식 대리점 "모아바이오"