다양한 분야에서 혁신적인 솔루션을 지원합니다.
제품 설명
CHIKV Envelope Antigen 2 (aa 339-692) [His]
제품 번호
DAGA-259
제품 특징
Nature
Recombinant
Tag/Conjugate
His
Alternative Names
Chikungunya virus envelope protein; CHIKV E2 protein; CHIKV E2; CHIKV Envelope Antigen 2; CHIKV
Purity
>95% , based on SDS PAGE
Format
Liquid
Concentration
Batch dependent - please inquire should you have specific requirements.
Buffer
In PBS with 8M Urea
Preservative
None
Storage
Keep it at 4˚C if used within a month. For long term storage, split it into small aliquots and keep at -80˚C. Avoid repeated freezing and thawing. The product will be expired one year after receiving if stored properly. Non-hazardous. No MSDS required.
Antigen Description
A member of the Togaviridae family, and Alphavirus genus, belonging to the Semliki Forest serological complex. CHIKV is a spherical enveloped virion that measures 60 to 70 nm in diameter, and contains a single-stranded, positive-sense RNA genome.
Keywords
Chikungunya virus envelope protein;CHIKV E2 protein;CHIKV E2;CHIKV Envelope Antigen 2;CHIKV
Citations
Have you cited DAGA-259 in a publication? Let us know and earn a reward for your research.
Background
Arthropods carry the Chikungunya virus (CHIKV), which belongs to the Alphavirus genus. These viruses are enveloped RNA viruses that can cause significant infections when they infect humans or animals. CHIKV is a positive-sense, single-stranded RNA virus that is around 70 nm in diameter and 11.8 kb in length. There are two open reading frames (ORFs) in the genome: the 3' ORF encodes structural proteins, whereas the 5' ORF encodes non-structural proteins (nsPs). The CHIKV envelope is made up of 240 nucleocapsid proteins, including spike glycoproteins E1 and E2. Viral infection is facilitated by the attachment of the E2 envelope protein to host cell receptors, which initiates receptor-mediated endocytosis. In order to allow the viral genome to enter the host cell, the E1 envelope protein must fuse the viral membrane with the membrane of the host cell. The E1 and E2 proteins combine to produce heterodimers, which aid in receptor binding and recognition. These proteins reorganize in the acidic environment of endosomes, revealing the E1 fusion loop that facilitates the fusion of the membranes of the virus and the host cell.
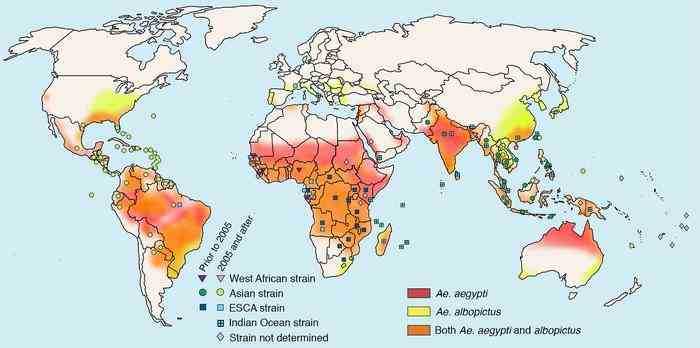 Figure 1. Geographic Distribution of Endemic CHIKV and Its Major Vectors (Source: Silva LA., et al., 2017)
Figure 1. Geographic Distribution of Endemic CHIKV and Its Major Vectors (Source: Silva LA., et al., 2017)
CHIKV is currently the most widespread alphavirus, and virologically, it is divided into three distinct lineages: the West African lineage, the East-Central-South African (ECSA) lineage, and the Asian lineage. Although the initial spread was limited to sub-Saharan Africa, the ECSA strain re-emerged in Kenya in 2004, rapidly spreading to the Indian Ocean islands, followed by Southeast Asia, India, and Southern Europe, leading to large-scale epidemics. This epidemic strain was later classified as the Indian Ocean lineage (IOL) and further expanded to temperate regions through the transmission by Aedes albopictus. After mosquito transmission, CHIKV can induce a syndrome that includes fever, rash, joint pain, and myalgia. The virus first replicates in host cells such as skin and fibroblasts before spreading through the lymphatic system to the bloodstream and dispersing throughout the body. The most typical signs of CHIKV infection include an initial fever, severe joint pain, rash, and muscular ache. Joint pain, in instance, can last for months or even years, resulting in a chronic illness. During the acute phase, CHIKV-infected individuals produce large virus loads, making them the predominant source of mosquito transmission. Although CHIKV has a low mortality rate, its capacity to develop persistent arthritic symptoms and high viral loads contributes to its broad dissemination, posing a substantial public health risk.
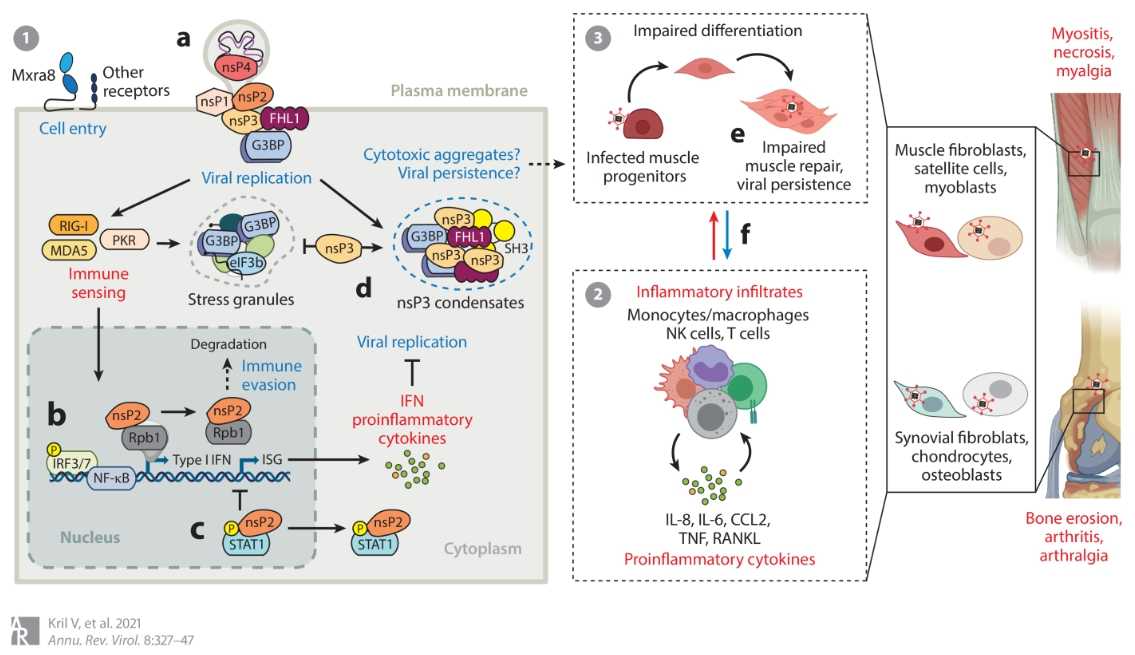 Figure 2. Hypothetical Model of Muscle and Joint Pathogenesis Induced by CHIKV (Source: Kri V., et al., 2021)
Figure 2. Hypothetical Model of Muscle and Joint Pathogenesis Induced by CHIKV (Source: Kri V., et al., 2021)
There are currently no particular antiviral medications or vaccines for CHIKV, therefore patient therapy is primarily symptomatic, with analgesics and anti-inflammatory drugs used to alleviate symptoms. Traditional mosquito control tactics have had minimal success in reducing CHIKV transmission, but they remain the principal preventive tool. In recent years, researchers have worked to produce effective antiviral medicines and vaccines for CHIKV. Ribavirin, an FDA-approved medicine, has shown promising results in clinical trials against CHIKV infection, but its wider use is limited due to serious side effects. Furthermore, studies have shown that monoclonal antibodies can neutralize the virus, avoiding or minimizing CHIKV infections. In experiments with mice and non-human primates, monoclonal antibodies have shown strong protective effects against the virus, even when used in the later stages of infection, reducing symptoms. Therefore, monoclonal antibodies are considered a potential direction for future CHIKV treatment. In vaccine development, although no official vaccines have been approved for market use, several candidate vaccines have entered clinical trials. These vaccines aim to provide long-term protection by inducing durable neutralizing antibodies, thus mitigating the global impact of CHIKV epidemics. In conclusion, CHIKV envelope proteins play a crucial role in viral infection, with the E2 protein being key in the interaction between the virus and host cells. In-depth study of the molecular mechanisms of CHIKV and the host immune response may result in more potent therapeutic and preventive strategies in the future, lowering the threat CHIKV poses to human health even though there aren't any particular antiviral medications or vaccinations available yet.
Chikungunya Virus E2 Protein (aa 339-692) [His]
ChikV E2 Protein (aa 339-692) [His]
Chikungunya Virus Envelope Protein 2 (aa 339-692) [His]
CHIKV E2 Antigen [His]
References
1. Silva LA., et al. Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127(3):737–749.
2. Kri V., et al.New insights into Chikungunya virus infection and pathogenesis. Annu Rev Virol. 2021;8:327–347.
References
Macrophage scavenger receptor 1 controls Chikungunya virus infection through autophagy in mice
COMMUNICATIONS BIOLOGY
Authors: Yang, Long; Geng, Tingting; Yang, Guang; Ma, Jinzhu; Wang, Leilei; Ketkar, Harshada; Yang, Duomeng; Lin, Tao; Hwang, Jesse; Zhu, Shu; Wang, Yanlin; Dai, Jianfeng; You, Fuping; Cheng, Gong; Vella, Anthony T.; Flavell, Richard. A.; Fikrig, Erol; Wang, Penghua
The vaccinia virus based Sementis Copenhagen Vector vaccine against Zika and chikungunya is immunogenic in non-human primates
NPJ VACCINES
Authors: Prow, Natalie A.; Liu, Liang; McCarthy, Mary K.; Walters, Kevin; Kalkeri, Raj; Geiger, Jillian; Koide, Fusataka; Cooper, Tamara H.; Eldi, Preethi; Nakayama, Eri; Diener, Kerrilyn R.; Howley, Paul M.; Hayball, John D.; Morrison, Thomas E.; Suhrbier, Andreas
CD Creative Diagnostics의 모든 제품을 만나보세요!
Products
Self-Assembled Monolayers Reagents
Protein-Based Fluorescent Nanoparticles
CD Creative Diagnostics - Official Distributor in South Korea "Morebio" 한국 공식 대리점 "모아바이오"